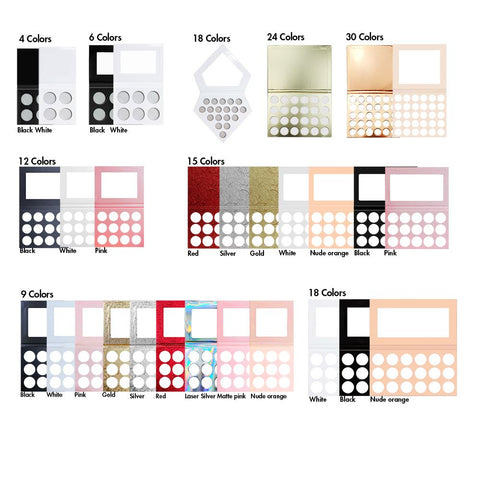
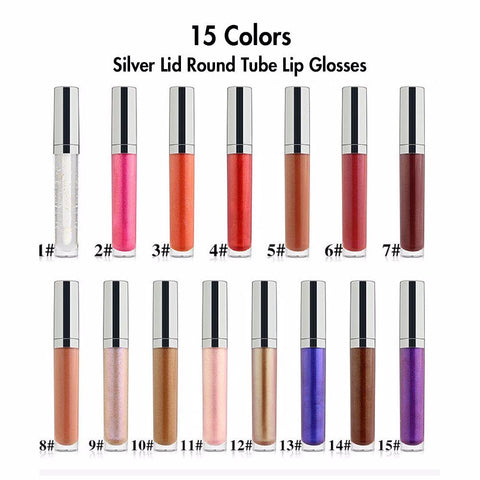
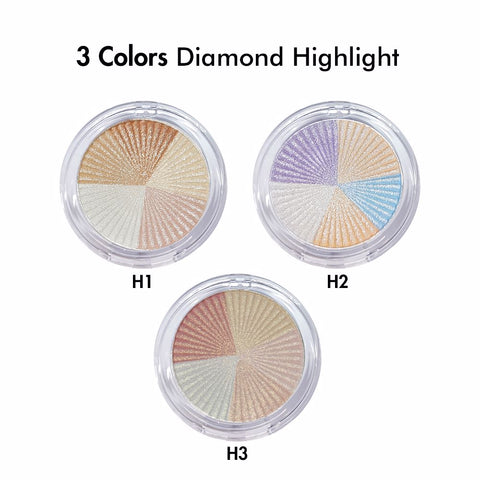
Supervision of cosmetics
In the United States, makeup are regulated by FDA as outlined in the food, drug and cosmetic act of the United States (FD &C act). The law requires that before entering the market, each cosmetic and personal care product and its ingredients must be verified for safety and must not contain any prohibited ingredients. However, in addition to complying with FDA regulations, most cosmetic manufacturers go beyond the legal requirements and adopt other safety procedures and technical standards developed by the cosmetic industry.
Us Food and Drug Administration (FDA)
The U.S. Food and Drug Administration (FDA) and the U.S. attorney general can take action against any company that sells unsafe cosmetics. The law imposes severe penalties on manufacturers of personal care products that do not meet these stringent safety standards, including confiscation, recall, fines and bans. In the past, the FDA has exercised this power to restrict or prohibit ingredients it considers unsafe.
It is the responsibility of cosmetic manufacturers to ensure that products comply with the law before they are sold. This process includes the analysis of cosmetic ingredient testing and safety data. If the manufacturer is unable to verify the safety of the product, the law requires the product to carry a clear warning. Check the FDA website for more information.
The role of FDA:
Prohibition or restriction of cosmetic ingredients for safety reasons
Authorization warning label
Inspection of production facilities
Issue a warning letter
Seizure of unsafe or misbranded products
Prohibition of illegal activities
Prosecution and violation of prison regulations
Regulation and approval of over the counter drugs (OTC) for cosmetics
Cooperate with cosmetic manufacturers to implement nationwide product recall
Collect samples for inspection as part of factory and import inspection
Research on personal care products and ingredients to solve safety problems
What FDA doesn't do:
Evaluate and register new cosmetic ingredients
Test and approve individual cosmetics
Cosmetic labeling requirements:
In addition to the food, drug and Cosmetic Act, the fair packaging and Labeling Act also empowers the FDA to require the sale of ingredient labels for cosmetics and personal care products to consumers. Detailed FDA regulations specify where and how ingredients must be listed on packaging. Find out more details about labeling regulations.
OTC drugs
The FDA also has the authority to regulate personal care products containing active ingredients from over-the-counter drugs (OTC). OTC drugs used in personal care products (including cosmetics) must be approved by FDA. Generally, a drug must obtain FDA pre marketing approval, or comply with final federal regulations, which set forth recognized conditions for safety and efficacy, and must not be mislabeled.
At present, some (but not all) OTC drugs (i.e. OTC drugs) sold before the start of OTC drug review (May 11, 1972) may be sold without specific approval, waiting for final rules and regulatory OTC drug reviews to be issued in accordance with current regulations. Once regulations covering specific categories of OTC drugs are finalized, they must be the subject of an approved new drug application (NDA) or comply with the appropriate federal regulations for OTC drugs.
The FD & C act defines some uses of drugs as "articles intended to diagnose, cure, alleviate, treat or prevent diseases" and "articles intended to affect the structure or the physical function of any other article (except food)" human or other animals ". The FD & C act does not recognize any such category as "cosmetic". Products can be drugs, cosmetics or a combination of the two, but according to the law, the word "drug makeup" has no meaning. Check the FDA website for more information. Examples of cosmetics over the counter drugs are:
Dandruff remover (e.g. shampoo)
Fluoride (e.g. fluoride in toothpaste)
Sunscreen (e.g. sunscreen)
Antiperspirant (e.g. deodorant)
Vcrp:
The voluntary cosmetic registration program (vcrp) is FDA's post marketing reporting system for cosmetics manufacturers, packers and distributors in the United States.
Vcrp helps FDA fulfill its mission of protecting consumers, provides valuable information to cosmetics manufacturers and distributors, and supports the safety assessment of cosmetic ingredients. The higher the degree of participation in the cosmetics industry, the better the effect of the plan, and the company benefits directly through the participation of the company.
It is important to note that vcrp is not an appearance approval procedure! Cosmetics do not require FDA pre marketing approval, mandatory business registration or ingredient reporting. It is the responsibility of the company to ensure that its cosmetics and ingredients are marked safely and correctly and in full compliance with the law.
In addition, vcrp is not a promotional tool. It is forbidden to use the registration number or record number of vcrp for promotion purposes. Check the FDA website for more information.
Cosmetic ingredient review (CIR) expert group
The CIR panel was launched in 1976 to support the FDA and the American Consumer Association (CFA), an independent, non-profit scientific body, to assess the safety of ingredients used in cosmetics in the United States. The group is made up of world-renowned scientists and physicians publicly nominated by consumers, scientific and medical groups, government agencies and industry. CIR thoroughly reviews and evaluates the safety of ingredients used in cosmetics in an open, unbiased and professional manner, and publishes its results in peer-reviewed scientific literature. FDA, CFA and the personal care products committee provide non voting contact to the panel and actively participate in the review and discussion process.
Personal care products Committee (PCPC)
The personal care products Council, formerly known as the cosmetics, toiletries and perfumes Association, is a leading national trade association in the cosmetics and personal care products industry and represents the most innovative name for beauty today. The Council provides voice to more than 600 member companies on scientific, legal, regulatory, legislative and international issues related to the personal care products industry. The Council is a leading and reliable source of information for and about the industry, and an advocate for consumer safety and continuous access to innovative products.
Code of consumer commitment
In 2007, the personal care products Council developed the consumer commitment code, which provides consumers, regulators and other interested parties with a clear outline of specific commitments and practices of cosmetics and personal care products companies to ensure the continued safety of all cosmetics.
The guidelines combine and strengthen practices that most companies have already adopted, such as current reporting to manufacturers, and include new practices, such as the safety information summary program, which provides FDA with information on cosmetic and ingredient safety upon request. The following is a summary of the main principles of the code; the full text can be read on the website of the personal care products committee.
Beispiel-Block-Zitat
Praesent vestibulum congue tellus bei fringilla. Curabitur vitae semper sem, eu convallis est. Cras felis nunc commodo loremous convallis vitae interdum non nisl. Maecenas ac est sit amet augue pharetra convallis nec danos.
Beispiel Absatztext
Praesent vestibulum congue tellus at fringilla. Curabitur vitae semper sem, eu convallis est. Cras felis nunc commodo eu convallis vitae interdum non nisl. Maecenas ac est sit amet augue pharetra convallis nec danos dui.
Cras suscipit quam et turpis eleifend vitae malesuada magna congue. Damus id ullamcorper neque. Sed vitae mi a mi pretium aliquet ac sed elitos. Pellentesque nulla eros accumsan quis justo at tincidunt lobortis denimes loremous. Suspendisse vestibulum lectus in lectus volutpat, ut dapibus purus pulvinar. Vestibulum sit amet auctor ipsum.